The Leader in Medical Device Assembly & Contract Manufacturing
Providien is a leading contract manufacturer of clinically superior medical devices. We provide advanced manufacturing solutions to the medical device and life sciences industries.
Providien has proven expertise with Class II and Class III, finished, medical device assembly. Utilizing our current world-class facility in Tijuana, Mexico, we are reducing time-to-market and costs, while always focusing on maintaining our rigorous quality standards and ensuring reliability.
In addition, through consistent investment from our parent company, Carlisle Medical Technologies, we are building a new state-of-the-art facility in the same business park as our existing facility. This new facility will have a wide range of capabilities, specializing in the development and manufacturing of our interconnect technologies business unit. By doing this, we are furthering our integration, shortening manufacturing timelines, and lowering overall production costs.
Advantages of Medical Assembly in Tijuana, Mexico
Providien’s “Nearshore” manufacturing location in Tijuana, Mexico is only 30 minutes from San Diego International Airport. This proximity offers significant advantages for our customers over other low-cost labor geographies making the transfer of your manufacturing line as seamless as possible.
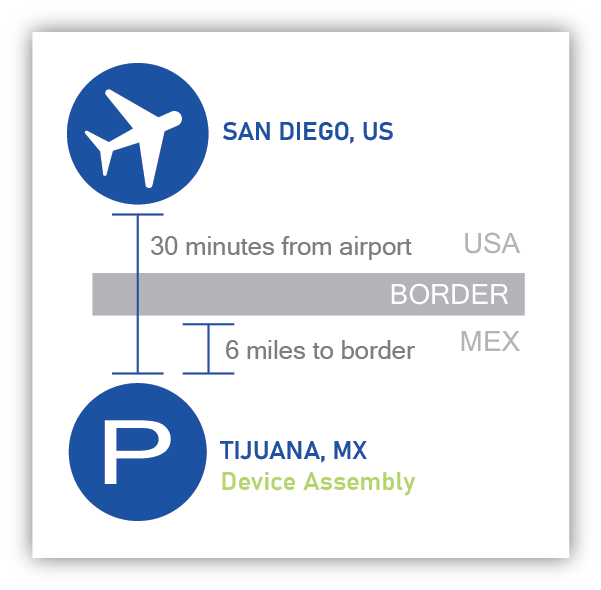
Providien’s Device Assembly’s “Nearshore” location may also provide you the following:
- Engineering and Customer Service support within a couple hours, if not the same time zone
- Product can be shipped to the United States in hours, not days
- Cost advantage over locations that have been impacted by recent Tariffs
- Ability for your team to travel to our facility in a few hours
Please let us know if you have any questions about Providien’s “Nearshore” offering, we would be happy to take a look at your next project and see if it may be a fit.
Our Capabilities in Tijuana
Providien Device Assembly (PDA), Located in Tijuana Mexico, has been in business for over 20 years, specializing in Medical Devices. Our 42,000 sq ft. facility includes 22,000+ sq ft ISO Class 8 Clean Room, expandable to 28,500 sq ft. and fluctuates around 800 employees.
Our experienced team has been manufacturing these medical devices for over 20 years. Our facility in Tijuana, Mexico is a state-of-the-art manufacturing and assembly facility.
Our robust validation and transfer protocols ensure a smooth launch for your finished medical device.
Medical Manufacturing Therapeutic areas of focus:
- Energy-Based Devices

- Complex Catheters
- RF Welded Bag Sets
- Oncology
- Tubing Sets
- Drug Delivery
- Disposable Infusion Pumps
Quality Certifications:
- FDA Registered
- ISO 13485: 2016
- Manufacturing of Class II and Class III Devices
- Japanese Certificate of Foreign Medical Device Manufacturer Accreditation
- Korean FDA Approved Supplier
Capabilities of Contract Manufacturing Tijuana:
- Solvent Bonding / UV Curing
- Ultrasonic Welding / RF Welding
- Catheter Tipping, Punching and Flaring
- Electromechanical Assemblies / Testing
- Soldering
- Laser Marking
- Micro Assembly / High Power Magnification
- Pad Printing
- Form Fill & Seal (FFS)
- Sterile Barrier Packaging / Sterilization Management
- Multiple Manufacturing Lines with expertise in Precise Assembly under Microscope
Catheter Assembly Equipment:
- RF Tipping Machine

- Magnified Vision Inspection
- Tunnel/Oven Curing Systems
- Vector Measurement System
- Pneumatic Dispenser
- Pull and Leak Testers
- Clamp Tipping Dilator
- Tray & Bar Sealer
Utilizing the local Tijuana Medical Device supply base:
- Expansive supplier network within medical device manufacturing in Mexico
- Over 150 Suppliers Managed
- Over 2,000 Components Sourced
- Supplier Scorecards
- Active Supplier Quality Auditing
- Approved Supplier List
- Local Sourcing
Third-Party Logistics:
- Cross Dock and Distribution
- Warehousing and Order Fulfilment
- Only Logistics Company to have U.S. and MX Customs – One Responsible 3PL
- Medical Device Expertise 13485 Certified
Our robust validation and transfer protocols ensure a smooth launch for your finished medical device.
Watch this video of an overview of our capabilities:
Medical Device Assembly and Manufacturing Capabilities in Mexico
Our main focus is on improving product quality and reliability while reducing time-to-market and costs. Providien has vast proven expertise.
Solvent Bonding
Solvent Bonding is a sealing process, solvent bonding bonds pieces of material using a strong solvent. The solvent used to bond the materials together works by softening and dissolving the material.
The surface molecules of the materials mix together while simultaneously dissolving. The surfaces form a permanent seal as the solvent evaporates.
Materials used with this technique are plastics and plastic tubing. In medical applications, solvent bonding seals IV tubing to ports and connectors. Heat can warp the tubes.
UV Curing
UV curing is an adhesive application process. Ultraviolet light and visible light create a reaction that generates a crosslinked network of polymers. This hardens liquid material to create a strong adhesive.
UV curing bonds dissimilar materials together. Materials such as plastics, glass, steel, or rubber bond together with this technique. UV curing is a low-temperature, high-speed process that is a great solution for many applications.
Providien has extensive experience with UV curing. We and commonly use the following grades of Loctite in our medical device assembly and manufacturing operations:
LOCTITE® 7701 Primer – A colorless, solvent-based liquid primer used to make low-energy surfaces suitable for bonding. Recommended for Difficult to bond substrates. Suitable for use in the assembly of disposable medical devices.
LOCTITE® 4061 – Transparent, colorless, low viscosity, ethyl-based instant adhesive. Particularly suitable for plastics and rubbers. ISO 10993 certified. Typical applications include the assembly of disposable medical devices.
LOCTITE® 4306 & 4307 – Designed for bonding applications that require very rapid fixturing, fillet cure, or surface cure. The UV light cure properties facilitate rapid curing of exposed surface areas, which minimizes blooming. This provides an alternative to solvent-borne accelerators. Suitable for use in the assembly of disposable medical devices.
LOCTITE® – colorless to slightly amber, transparent, isopropanol amine solvent-based liquid cyanoacrylate activator used where increased cure speed of LOCTITE cyanoacrylate adhesives is required.
LOCTITE® 4305 – Transparent, pale green, UV/V is light-cure cyanoacrylate adhesive that also cures in the presence of surface moisture. Provides outstanding plastic bonding capabilities and super-fast surface cure; tack-free time up to 5 secs and fixture time 2 secs. Ideal for plastics but also suitable for bonding ceramics and metals.
The most common plastics we work with are:
Acrylic
- A polymer of methyl methacrylate (PMMA), which is a transparent commodity thermoplastic and is useful for applications that require transparency, clarity, or impact resistance.
ABS
- Acrylonitrile butadiene styrene (ABS) is a common thermoplastic used in applications that require impact resistance, structural strength and stiffness, chemical resistance, excellent high and low-temperature performance, great electrical insulation properties, and or easy to paint and glue
NYLON
- A tough, easy-to-machine thermoplastic that is used for its great wear properties. Notable characteristics include high abrasion, impact resistance, high compressive strength, great electrical insulation properties, mechanical stability/hardness, high mechanical damping properties, fatigue resistance, and FDA approved.
PC
- Polycarbonate (PC) is a transparent thermoplastic polymer that is widely used as an engineering plastic due to its properties, such as, high performance, high impact strength, high dimensional stability, good electrical properties, and more.

PC/ABS
-
-
- A blend of Polycarbonate and acrylonitrile butadiene styrene. Uses cases vary depending on the ratio of the blend, but this is often used as a more cost-effective alternative to PC. It is also colorable and printable, allowing for more freedom regarding design requirements.
-
PET
- Polyethylene terephthalate (PET or PETE), belonging to the polyester family, is a general-purpose thermoplastic that can be semi-rigid to rigid, depending on processing. Notable properties include mechanical/thermal/chemical resistance, dimensional stability, recyclability, high flexibility, and more.
Friction Fit Assembly
Friction fit is fastening between two parts. Friction pushes parts together, rather than by any other means of fastening. Friction Fit can also be referred to as Press Fit or Interference Fit.
Radio Frequency Welding
R.F. or radio frequency welding is the process of bonding together two materials. R.F. uses electromagnetic energy to bond the materials together. An electric field causes polar molecules to shift. This movement creates heat.
When enough heat forms, the molecules begin bonding to each other. No outside heat source gets used in this process. The weld finishes by applying pressure to the bonds to seal them together.
R.F. Welding, in particular, creates a complete, air-tight seal. This is ideal in medical device assembly. R. F. Welding creates bonds between many different materials, including:
PET – Polyethylene terephthalate (PET or PETE)
PETG – Polyethylene terephthalate glycol, known as PETG or PET-G
PVC – Polyvinyl chloride is one of the most commonly used plastic polymers. It can be rigid and flexible, while being lightweight, durable, low cost, and easy to process
TPU – Thermoplastic polyurethanes
PC – Polycarbonate (PC) plastics
Catheter Tipping, Punching And Flaring
Catheter tipping is any secondary process performed on a catheter shaft or tip. It uses heat to make a rounded tip on the end of a tube. A rounded head makes insertion easier. Typically, radiofrequency heats the end of the tube until it is malleable. Another tube used as a mold presses into the tube. This creates the rounded edge.
Pad Printing
Pad printing is a printing process that includes transferring a 2-D image onto a 3-D object. This includes labels on catheters and units of measure on syringes. These objects have small surface areas, requiring precision printing.
Pad printing comes in handy for these projects. It can effectively print on small objects. Regular printing cannot mark or brand uniquely shaped objects. Pad printing uses special techniques and machines to label such materials.
Common pad printing applications include:
- Tube Marking

- Hole Punching
- Hub Printing
- Rigid Plastic Printing
- Marker Band Printing
- Micro-Catheter Printing
- Logo Applications
Hot Stamping
Hot stamping is a dry printing method of lithography. Pre-dried ink or foils get transferred to a surface at high temperatures and is common for plastics.
A “die” heats up above the product to be marked. A roll-leaf foil gets placed between the two. When the hot die presses down, it transfers the mark onto the product.
Since all products are dry, the process does not cause pollution.
The silicone or metal dies have fine detail that get transferred to the product. The foil acts as an adhesive.
They have multiple layers that include a color base, adhesive base, and release base.
Sewing
Sewing can have uses for specific medical device manufacturing operations. Primarily, medical sewing applies to devices that have cloth. Manual sewing or machine sewing are both used in these applications.
Tool & Assembly Fixture Development
Providien has a breadth of experience developing tooling and fixtures for a wide range of applications. Providien has extensive experience with designing tools and fixtures to improve the manufacturing process.
This includes tools and fixtures that can reduce cycle time, improve quality, reduce scrap and provide enhanced ergonomics for the assembly technicians. Providien’s Green and Black Belt Engineers are developed through the Providien Academy and are experts in the process of tool and fixture development to improve your medical device assembly manufacturing.






